Imagine a complex web of collaboration, precision, and insight coming together to drive medical advancements – that’s where the role of a Contract Research Organization (CRO) comes into play. Curious to know how these entities operate behind the scenes, seamlessly supporting the development of new treatments and therapies? Let’s delve into the intricate workings of a CRO, shedding light on their crucial contributions to the field of medical research. Read CRO In India
Role of CROs in Research
- In research, Contract Research Organizations (CROs) play a pivotal role in facilitating and executing various aspects of clinical trials and studies. CROs are responsible for overseeing the collection, management, and analysis of data during clinical trials. They ensure that data collection processes adhere to strict regulatory compliance standards set forth by governing bodies to guarantee the integrity and reliability of the research findings. By meticulously monitoring data collection procedures, CROs help maintain the quality and accuracy of the information gathered throughout the trial.
- In addition to data collection, CROs also focus on regulatory compliance to ensure that all aspects of the clinical trial adhere to the necessary guidelines and regulations. This involves thorough documentation and reporting to regulatory authorities to demonstrate that the trial is conducted ethically and within the established legal framework. Compliance with regulations is crucial in safeguarding the well-being of trial participants and upholding the credibility of the research outcomes. CROs serve as crucial partners in navigating the complex landscape of regulatory requirements to ensure the successful execution of clinical trials.
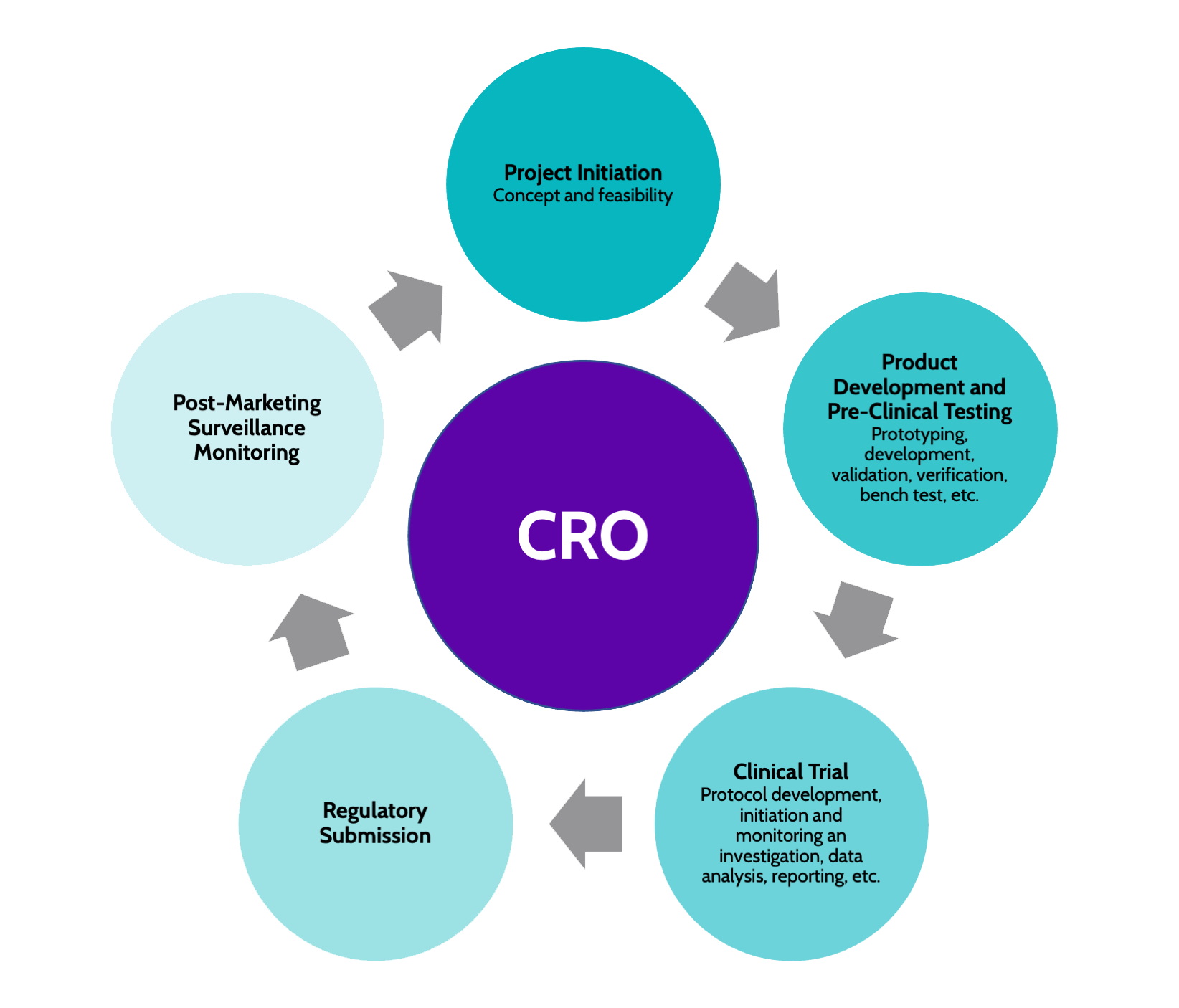
Services Offered by CROs
- Contract Research Organizations (CROs) typically provide a range of specialized services tailored to support the efficient execution of clinical trials and research studies. These services encompass various crucial aspects of the research process. CROs excel in data analysis, where they utilize advanced analytical tools to interpret and derive meaningful insights from the collected data. This capability ensures that the findings are accurately interpreted and communicated to stakeholders.
- Moreover, CROs play a pivotal role in ensuring regulatory compliance throughout the research process. They possess in-depth knowledge of the regulatory requirements governing clinical trials and research studies, thus assisting in navigating the complex regulatory landscape effectively. By keeping abreast of the latest regulatory developments, CROs help researchers maintain compliance and adhere to the necessary protocols.
Process of Clinical Trials
- How does the intricate process of conducting clinical trials unfold, guiding researchers through each meticulously planned step towards valuable scientific insights and regulatory approval?
- Clinical trials begin with the development of a study protocol outlining the research objectives, methodology, and participant eligibility criteria. Recruitment strategies are then implemented to enroll suitable participants, ensuring the study’s success and reliability of results.
- Once participants are enrolled, data collection begins, with researchers closely monitoring and documenting all relevant information. Data analysis plays a crucial role in clinical trials, involving the examination of collected data to draw conclusions and evaluate the study outcomes.
- Statistical methods are applied to interpret the data accurately and determine the effectiveness and safety of the tested intervention. Throughout the process, adherence to ethical standards and regulatory requirements is paramount to safeguard participant rights and ensure the validity of the research findings.
Collaboration With Sponsors
- As researchers progress through the meticulous steps of conducting clinical trials, collaboration with sponsors becomes a pivotal aspect in ensuring the study’s progression and success. Sponsor relationships are crucial in defining the scope of the trial, aligning on objectives, and establishing clear communication channels. Effective sponsor relationships are built on transparency, trust, and mutual understanding of project goals.
- Project management plays a significant role in fostering strong sponsor relationships. Clear project planning, including timelines, milestones, and deliverables, helps maintain alignment between the CRO and the sponsor. Regular updates, progress reports, and risk assessments enhance communication and provide sponsors with the necessary insights into the trial’s status.
- Successful collaboration with sponsors requires proactive problem-solving and a solutions-oriented approach. CROs must be agile in addressing challenges, managing resources efficiently, and adapting to changing circumstances. By prioritizing sponsor relationships and effective project management, CROs can navigate clinical trials successfully, ensuring adherence to timelines and meeting study objectives.
Impact on Medical Innovation
- The role of a CRO in driving medical innovation through clinical trials is crucial in advancing healthcare research and treatment options. CROs play a significant part in overcoming innovation challenges by providing expertise, resources, and infrastructure to facilitate the development of new medical solutions.
- These organizations help navigate the complex landscape of regulatory hurdles by ensuring that clinical trials adhere to stringent guidelines and requirements set forth by regulatory bodies. By efficiently managing the various aspects of clinical trials, CROs enable medical innovators to focus on research and development, ultimately accelerating the pace of bringing new treatments to patients.
- Innovation in the medical field often faces challenges such as high development costs, lengthy approval processes, and stringent regulatory requirements. CROs play a vital role in addressing these challenges by streamlining the clinical trial process, optimizing resource allocation, and ensuring compliance with regulatory standards. Through their expertise and experience, CROs contribute significantly to overcoming innovation barriers, ultimately driving advancements in healthcare that benefit patients worldwide.
Conclusion
In conclusion, CROs play a crucial role in driving medical innovation through their expertise in conducting clinical trials. By offering a wide range of services to sponsors, CROs facilitate the research process and help bring new treatments to market more efficiently. Their collaborative approach with sponsors ensures that trials are conducted effectively and in compliance with regulations. Overall, the impact of CROs on advancing healthcare through research can’t be overstated.
Leave a Reply